
Admissions consulting
We are engineers, physicians and QM experts whose in-depth expertise is based on a large number of international development, manufacturing and approval projects. Our customers benefit from this expertise.

Legal manufacturer
We are engineers, physicians and QM experts whose in-depth expertise is based on a large number of international development, manufacturing and approval projects. Our customers benefit from this expertise.

Clinical approval – CRO
We are engineers, physicians and QM experts whose in-depth expertise is based on a large number of international development, manufacturing and approval projects. Our customers benefit from this expertise.

Process consulting
We are engineers, physicians and QM experts whose in-depth expertise is based on a large number of international development, manufacturing and approval projects. Our customers benefit from this expertise.

QM Consulting
We are engineers, physicians and QM experts whose in-depth expertise is based on a large number of international development, manufacturing and approval projects. Our customers benefit from this expertise.
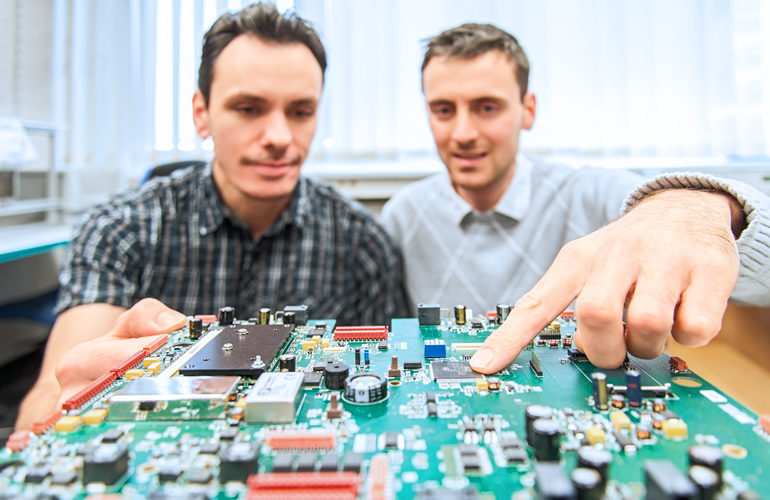
Quality management
seleon is experienced in the production of highly specialized devices. Qualified and experienced employees as well as comprehensive, modern equipment guarantee the production of medical technology in perfection.
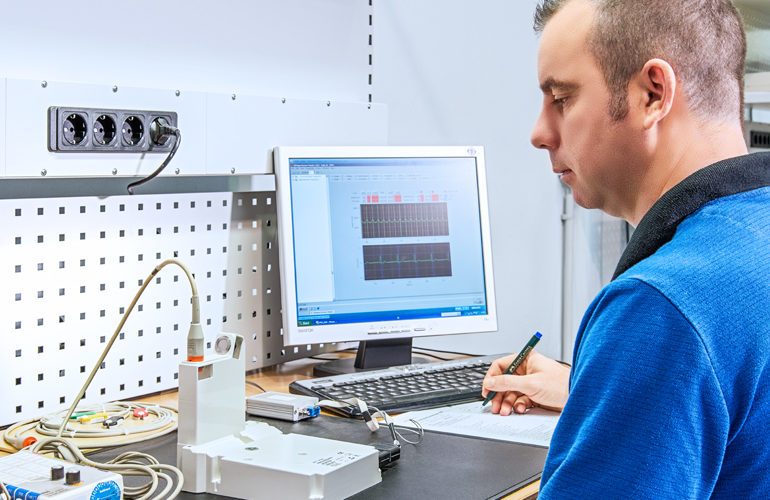
Manufacturing and testing equipment
seleon is experienced in the production of highly specialized devices. Qualified and experienced employees as well as comprehensive, modern equipment guarantee the production of medical technology in perfection.

Special systems
seleon is experienced in the production of highly specialized devices. Qualified and experienced employees as well as comprehensive, modern equipment guarantee the production of medical technology in perfection.
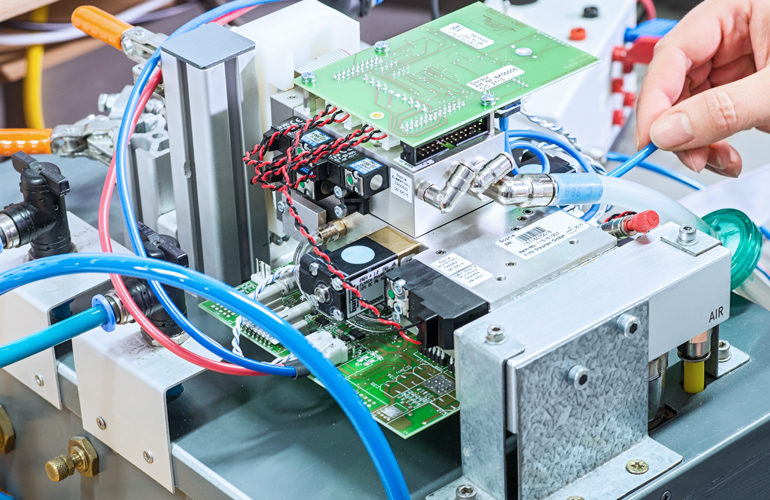
Medical technology production
seleon is experienced in the production of highly specialized devices. Qualified and experienced employees as well as comprehensive, modern equipment guarantee the production of medical technology in perfection.

Supply-Chain-Management
seleon is experienced in the production of highly specialized devices. Qualified and experienced employees as well as comprehensive, modern equipment guarantee the production of medical technology in perfection.

Documentation / Testing
We turn your ideas into products that are at the forefront of medical technology. You have access to all the specialist areas required for the development of complex medical technology systems.

Mechanics / Mechatronics
We turn your ideas into products that are at the forefront of medical technology. You have access to all the specialist areas required for the development of complex medical technology systems.

Software / Firmware
We turn your ideas into products that are at the forefront of medical technology. You have access to all the specialist areas required for the development of complex medical technology systems.
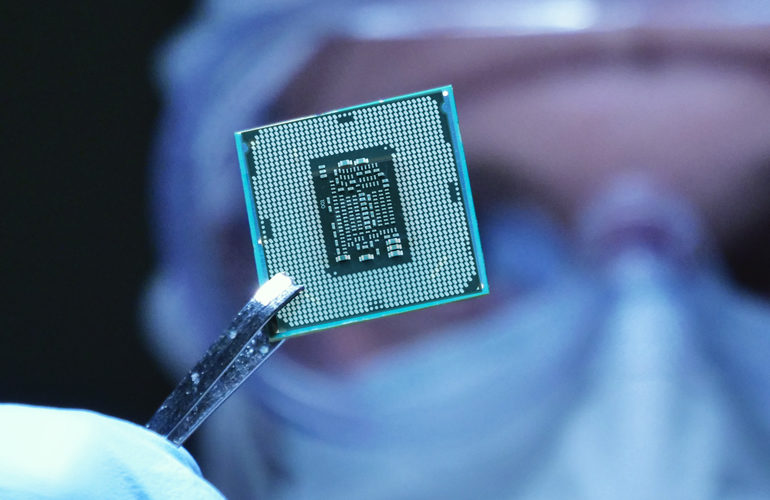
Electronics
We turn your ideas into products that are at the forefront of medical technology. You have access to all the specialist areas required for the development of complex medical technology systems.

System development
We turn your ideas into products that are at the forefront of medical technology. You have access to all the specialist areas required for the development of complex medical technology systems.

Project Management
We turn your ideas into products that are at the forefront of medical technology. You have access to all the specialist areas required for the development of complex medical technology systems.

Production
seleon is experienced in the production of highly specialized devices. Qualified and experienced employees as well as comprehensive, modern equipment guarantee the production of Medical Technology in perfection.

Consulting
We are experienced engineers, doctors and QM experts whose in-depth expertise is based on a multitude of international projects in the areas of development, manufacturing and approval. Our customers benefit from this expertise.

Development
We turn your ideas into products that are at the forefront of Medical Technology. You have access to all the specialist areas required for the development of complex Medical Technology Systems.

